-
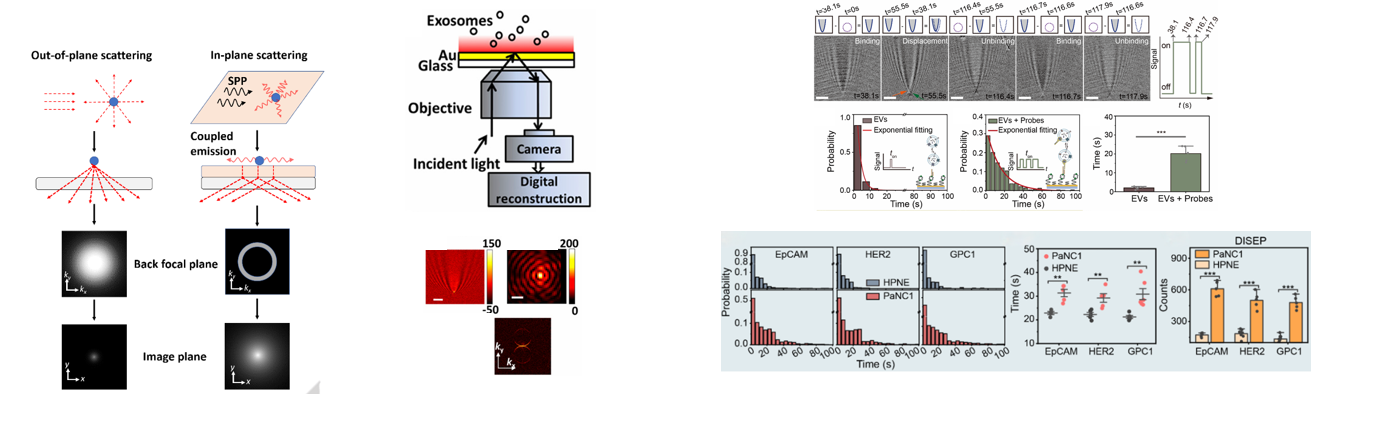
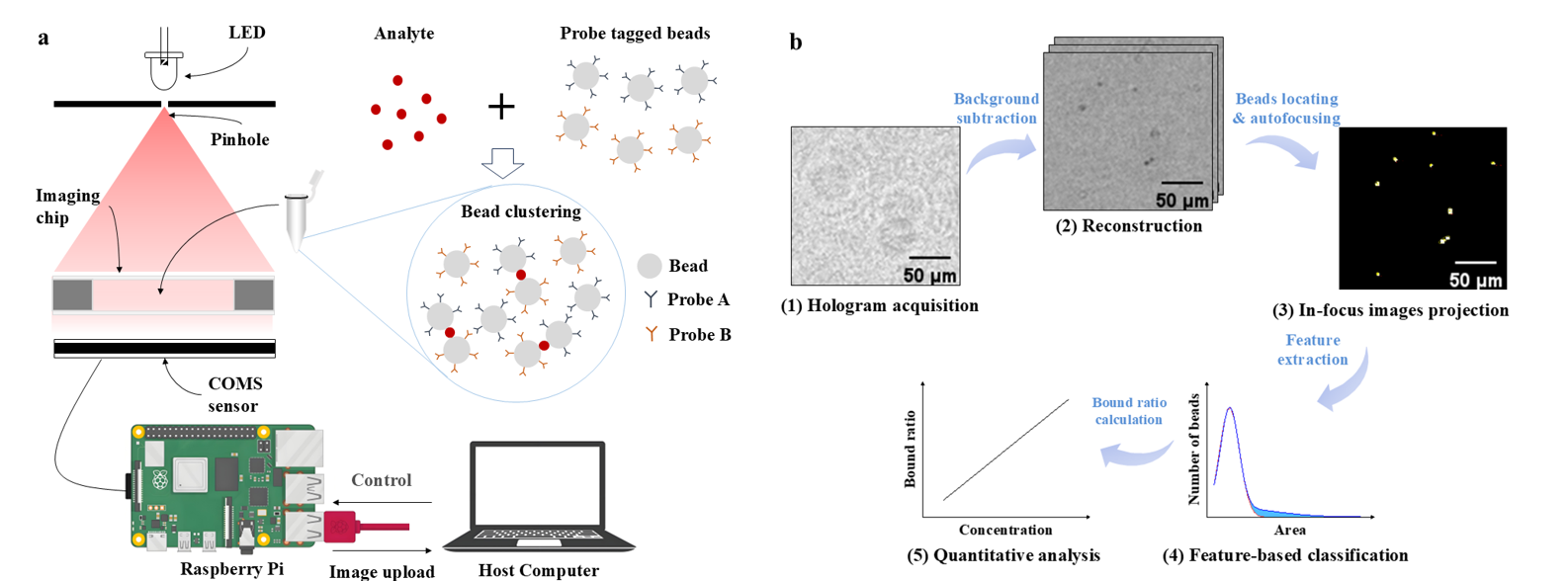
① Exosome detection
-
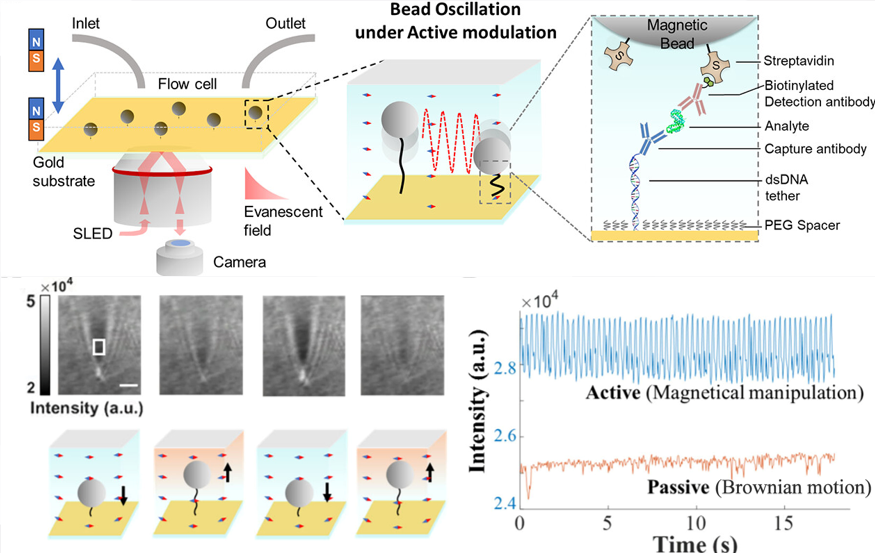
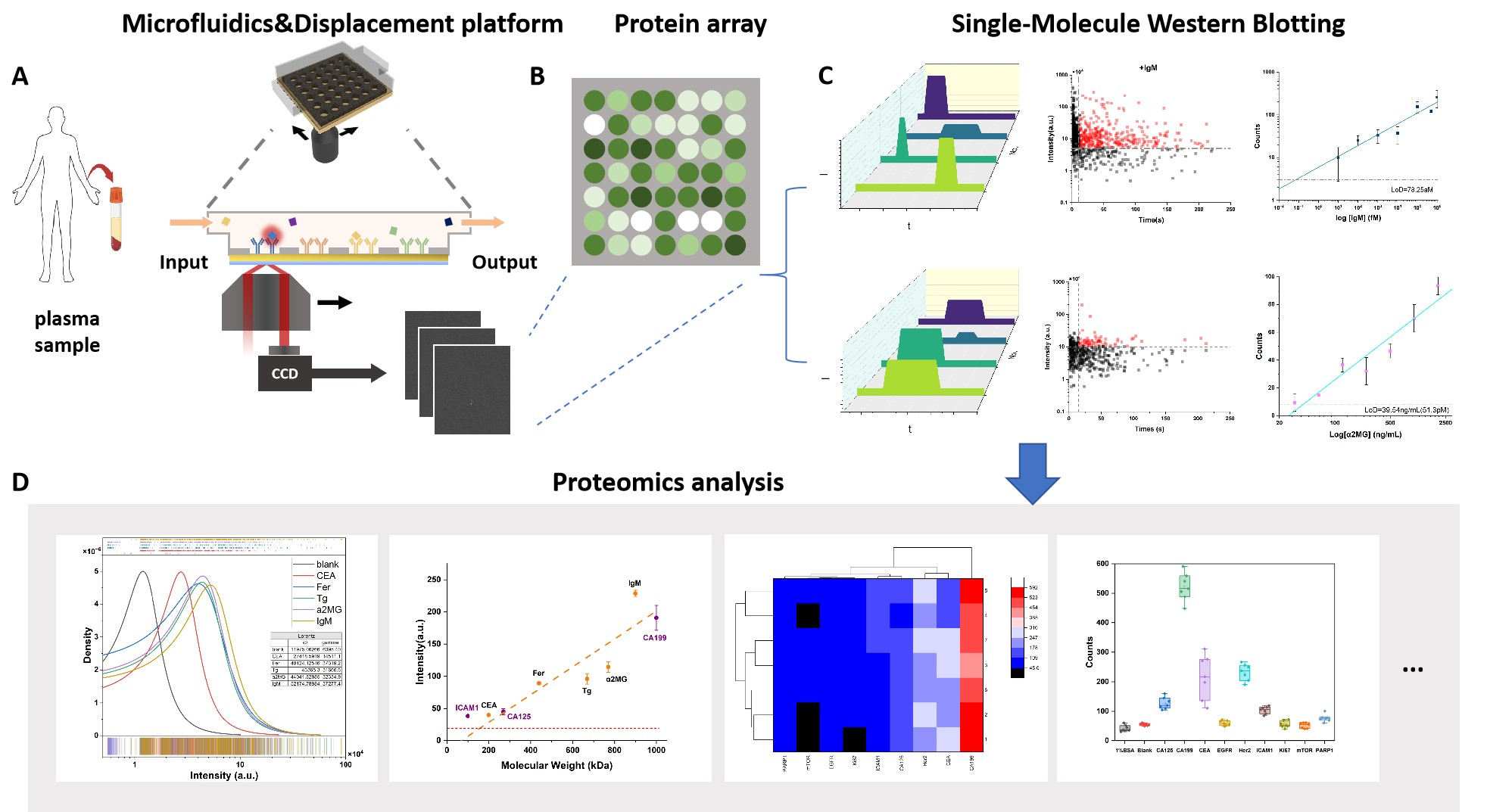
② Ultra-sensitive immunoassay
-
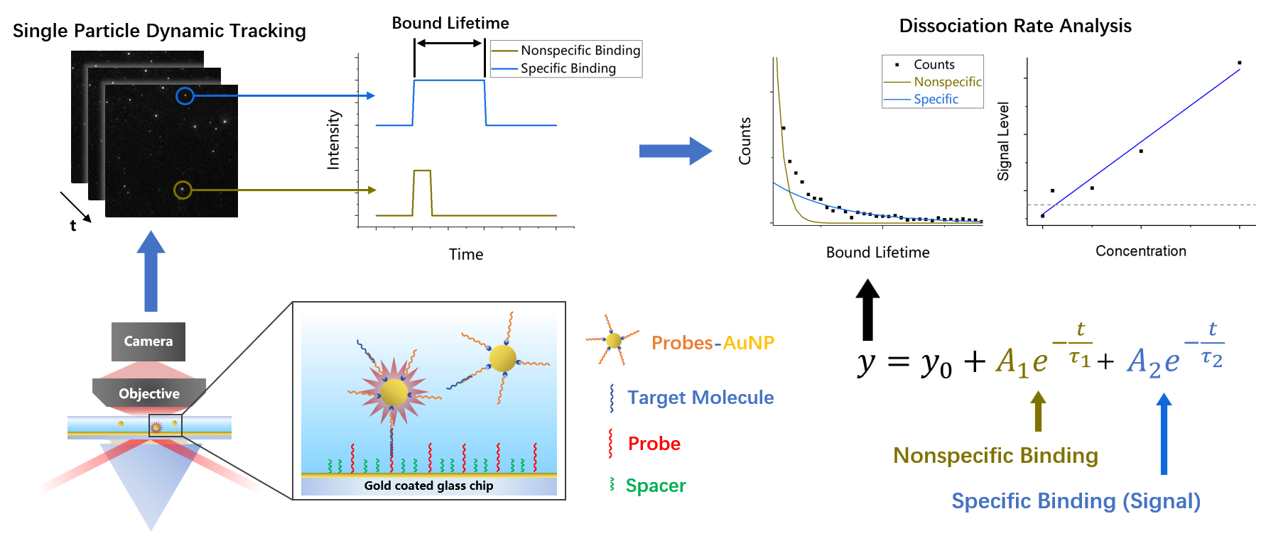
③ Molecular binding kinetics
-
Organoids are multi-cell clusters formed by three-dimensional culture of stem cells or tumor cells in vitro. Its growth process mimics the process of human development or in vitro organ regeneration. Tracking and analyzing the formation process of organoids can help study the potential mechanisms of human development and organ regeneration, and promote research in basic biology.
Based on multi-angle illumination lens-free imaging technology, we have designed a three-dimensional imaging system with a large measurement range, single-cell resolution, label-free, small volume (placed in an incubator), and fast imaging speed. Currently, we are dedicated to multimodal organoid analysis, including morphological and membrane protein imaging -
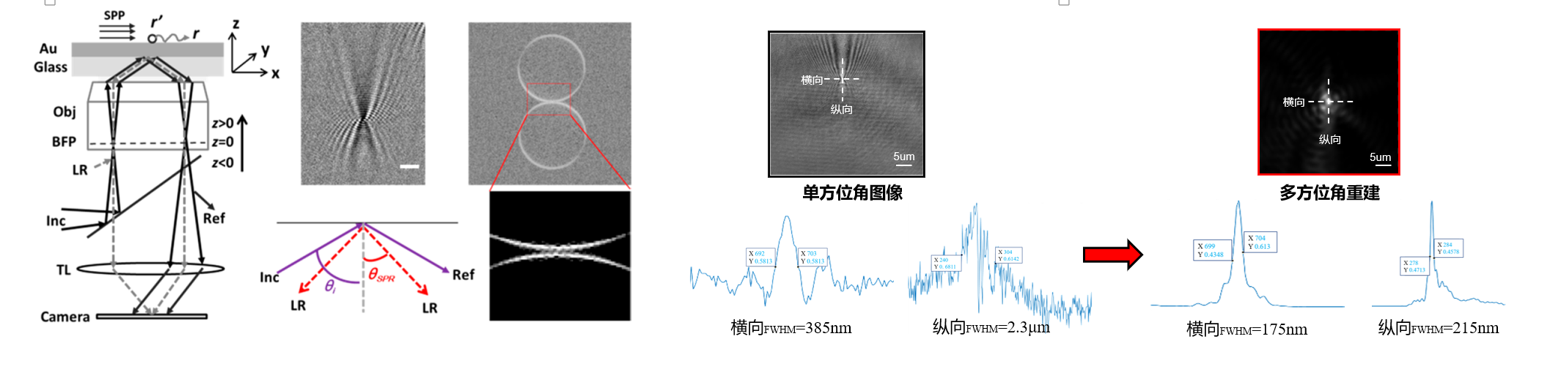
Traditional plasma resonance microscopy (SPRM) is widely used in the fields of biology, chemistry, etc. due to its advantages such as no labeling and high sensitivity. However, the classic "parabolic" images obtained by traditional SPRM have problems of insufficient spatial resolution and anisotropy.
To address the drawback of insufficient spatial resolution of SPR "parabolic" images, our group, based on its anisotropic characteristics, have optimized the imaging optical path by using multi-angle illumination. Combined with the development of deconvolution image reconstruction processing algorithms and optical aperture synthesis algorithms, we enabled the image to retain more high-frequency information, achieve super-resolution imaging, and break through the Rayleigh diffraction limit.